Autophagy, a major metabolic regulator involved in AML therapy resistance Malgré l’efficacité des chimiothérapies et l’arrivée de thérapies ciblées, les rechutes restent fréquentes et le pronostic vital des patients atteints de Leucémies Aiguës Myéloïdes (LAM) faible. Il est maintenant admis que la résistance aux traitements des cellules LAM nécessite une adaptation de leur métabolisme. Nous […]
16/06 : LE 13H DE MAUDE LE GALL @ RESTORE 🗓 🗺
Gastric and intestinal adaptations in response to bariatric surgery and their contribution to glycemic improvement The team gathers physiologists of the gastro-intestinal tract, basic scientists and clinicians (digestive surgeons, gastroenterologists and nutritionists) to develop basic and transitional researches to study gastrointestinal adaptations in response to obesity, surgical remodeling, and nutritional status (undernutrition / malnutrition). More […]
09/06 : LE 13H DE JULIE BATUT @ RESTORE 🗓 🗺
From olfactory organ formation using embryonic, mathematical and microfluidic models to a PhD student mentoring programme with CBI and Femmes & Sciences responses? Julie Batut est biologiste, spécialiste de la neurogenèse ainsi que de l’identité et la forme des cellules au cours du développement embryonnaire. Chercheuse au CNRS, elle étudie la mise en place de […]
21/04 : LE 13H DE MARIE ADLANMERINI @ RESTORE 🗓 🗺
Hypothalamic REV-ERB nuclear receptors control diurnal leptin sensitivity and circadian period: implications for diet-induced obesity Résumé de la présentation : Circadian disruption, as occurs in shift work, is associated with metabolic diseases often attributed to a discordance between internal clocks and environmental timekeepers. REV-ERB nuclear receptors are key components of the molecular clock, but their […]
17/03 : LE 13H DU PLATEAU CYTOMÉTRIE @ RESTORE 🗓 🗺
Présentation du nouvel analyseur / trieur de cellules spectral Aurora CS Cytek. Grâce à l’obtention d’un financement européen REACT-EU , le plateau de Cytométrie –TRI Genotoul de l’I2MC accueille depuis fin décembre 2021 un nouvel analyseur / trieur de cellules spectral Aurora CS Cytek. Ce nouvel équipement ouvert à la communauté toulousaine, combine les capacités d’analyses multiparamétriques de la […]
24/02 : LE 13H D’EMILIANO RICCI @ RESTORE 🗓 🗺
Quantitative mass spectrometry of affinity purified ribosomes uncovers ribosome interactome specialization during viral infection Emiliano Ricci est lauréat d’une ERC STARTING GRANT « RIBOINFLAM » sur l’Évaluation du rôle des ribosomes et de la traduction de l’ARNm dans la formation de la réponse inflammatoire. Résumé de sa présentation : Viruses are obligate intracellular parasites that rely on the host […]
14/04 : LE 13H DE NADINE LAGUETTE @ RESTORE 🗓 🗺
STING orchestrates the crosstalk between polyunsaturated fatty acids metabolism and inflammatory Nadine Laguette a été distinguée de la médaille de bronze du CNRS en 2021 et a été lauréate d’une ERC STARTING GRANT. Son équipe étudie les mécanismes moléculaires qui régulent l’inflammation chronique et ses conséquences physiopathologiques. Ses travaux visent à identifier la nature, la […]
25/11 : LE 13H DE LAURENT ALRIC @ RESTORE 🗓 🗺
Interactions entre malades, services cliniques et laboratoires de recherche Les interactions entre les malades, les services cliniques du CHU et les laboratoires de recherche sont nécessaires. Elles peuvent passer par la mise à disposition d’échantillons issus de malades ou des innovations thérapeutiques. Il sera donné des exemples comme la constitution d’une bio banque, la genèse […]
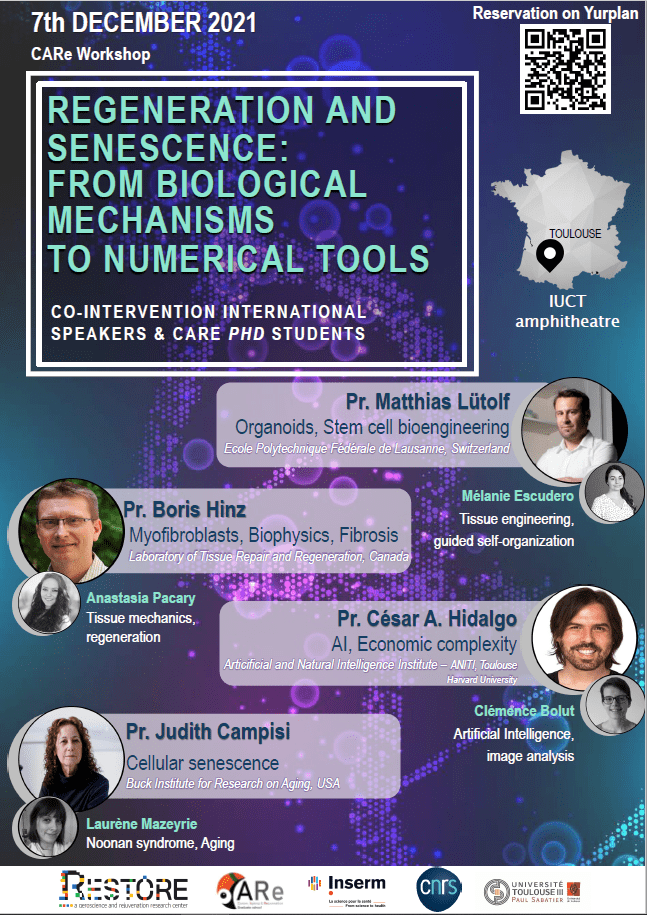
Séminaire CARe à RESTORE
Les étudiants CARe de l’institut RESTORE organisent un séminaire sur le thème de la régénération et de la sénescence : Regeneration and Senescence: From biological mechanisms to numerical tools 7 décembre 2021 Amphithéâtre IUCT, Oncopole (Toulouse) Nous aurons l’honneur de recevoir des chercheurs internationaux prestigieux tels que Judith Campisi, César A Hidalgo, Boris Hinz et […]
23/09 : LE 13H DE SOPHIE LE GONIDEC @ RESTORE 🗓 🗺
Présentation de l’activité et des expertises du service Phénotypage du CREFRE Au sein du Centre régional d’exploration fonctionnelle et de ressources expérimentales (CREFRE), le service Phénotypage propose un ensemble de prestations pour l’exploration fonctionnelle des modèles murins. Notre équipe accompagne la recherche toulousaine et nationale depuis 15 ans. Selon le besoin de chaque équipe et […]